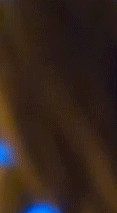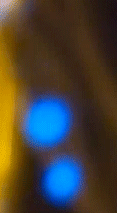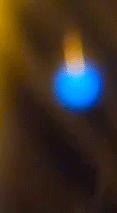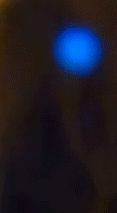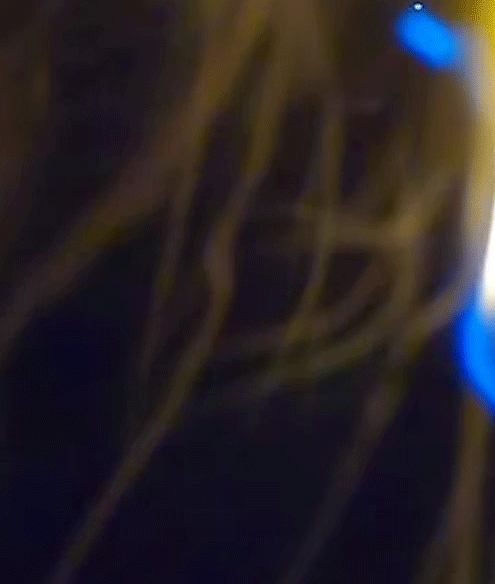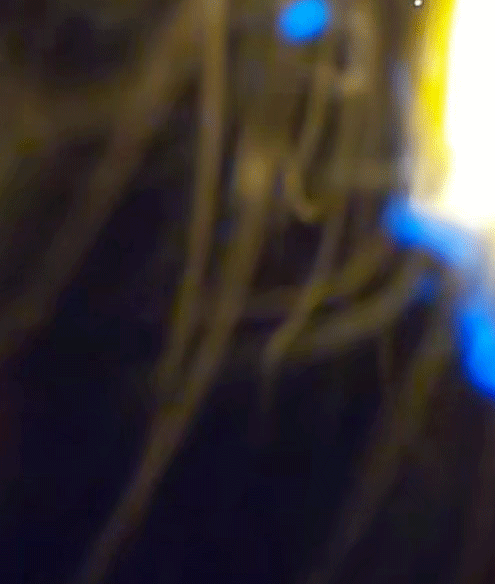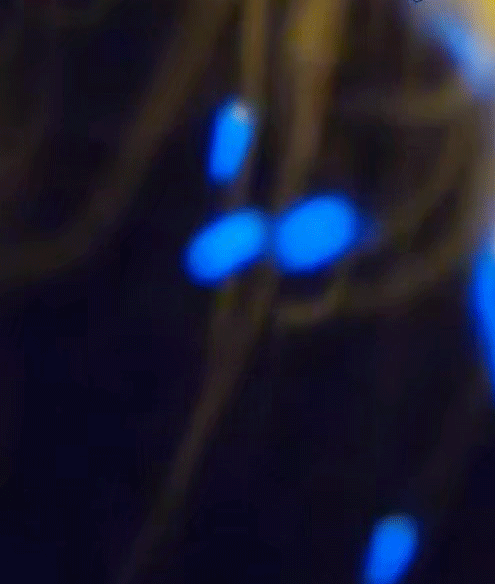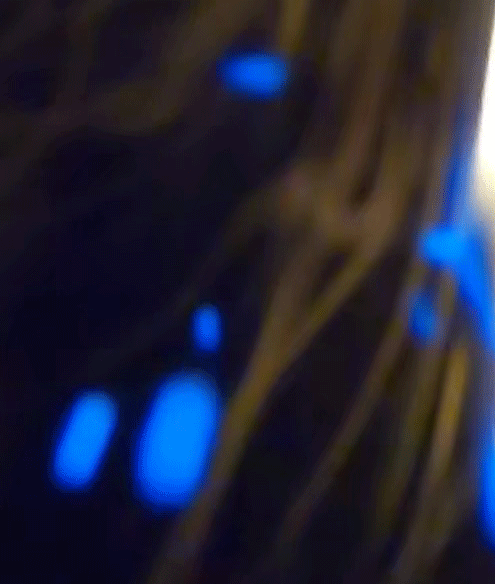A Spark
Quantum Phenomenon in a Candle Experiment

Today, lets embark on a journey, exploring an experiment that challenges our conventional understanding of where quantum effects can occur. In this presentation, we will delve into a seemingly ordinary candle that, under specific conditions, exhibits extraordinary behavior indicative of quantum phenomena.


The candle and the additive
At the heart of our experiment lies an ordinary paraffin wax candle, but with a twist. The wick of the candle is coated with a special organic additive. When lit, this additive plays a crucial role in initiating the quantum phenomena we observe.

Introducing the Quantum candle

What did we just witness?
What we have just witnessed is a fascinating display of plasma generation in a seemingly ordinary setting - a candle flame. The special organic additive on the candle's wick, when lit, facilitates the ionization of the atoms, turning them into a plasma state. Plasma is the fourth state of matter, alongside solids, liquids, and gases.
It consists of ions and free electrons and is commonly found in stars and lightning. In our experiment, the plasma state manifests as bright, colorful sparks ejected from the candle flame. Their blue color suggests a higher energy transition, as blue light corresponds to a higher frequency (and thus higher energy) within the visible spectrum.


Blue is quantum
The blue color of the sparks you've seen is a direct manifestation of a fundamental principle of quantum mechanics: energy quantization. In the quantum world, the energy of particles isn't continuous, but 'quantized', meaning it can only take on certain discrete values. When an electron in an atom makes a transition from a higher energy level to a lower one, it emits energy in the form of light. The energy (and therefore color) of this light corresponds to the energy difference between the two levels.
Blue light corresponds to a higher frequency, and therefore higher energy, within the visible spectrum. So, when you see blue sparks, you're witnessing electrons making higher energy transitions, emitting blue light as they return to a lower energy state. It's a remarkable thing, where quantized sparks emitted from a simple candle flame can provide a window into the world of quantum mechanics and the behavior of particles at the macroscopic level.
The Sparks are Quantum.

The Quantum Candle
Experiment